An Electrochemical Switch For Controlling The Flammability Of Liquid Fuels
Full Description

High speed camera imaging of the electrochemical activation and deactivation of the combustion process
Background
Liquid fuels do not combust in the liquid phase, rather they vaporize and combustion occurs due to the rapid oxidation of the free fuel molecules in the vapor phase. Ignition is basically a method to volatilize enough fuel to the flash point so that the heat feedback from the flame can self sustain the cobustion. Therefore, the typical way to extinguish a flame is to remove the oxygen source (air) from the flame front. Room temperature ionic liquids (RTILs) are a special class of compounds that are non-flammable and are often used as safe electrolytes for metal batteries.
Technology
By considering the possibility of dynamically manipulating the volatility of the liquid fuel, rather than removing the oxygen, Prof. Zachariah, and his team have developed a novel technology that is a paradigm shift and offers the potential to make a "safe fuel". The team has shown that RTILs that do not ignite when exposed to thermal shock or when contacted with a flame - can be made to electrochemically decompose to reactive volatile species which can ignite and sustain a flame. The developed technology can also extinguish the flame by decreasing its volatility.
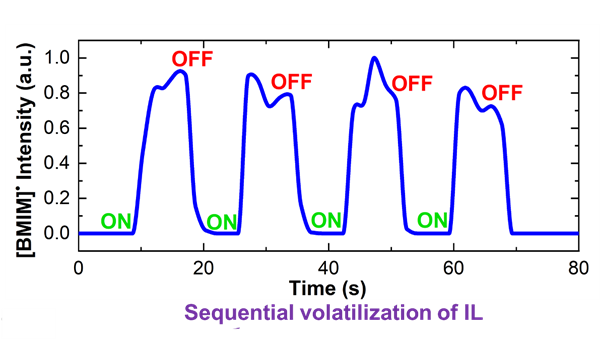
Graph showing the sequential volatilization of 1-butyl, 3-methyl imidazolinium perchlorate - [BMIM]+[ClO4]-.
Advantages
The significant benefits and unique aspects of this technology are:
- Ability to store an involatile energetic fluid as a non-flammable fuel.
- Dynamically manipulate the volatility to turn on or off, to ignite and sustain the flame - like an electrochemical switch.
- Negates the need for complex interventions in tackling a flame.
Suggested uses
Potential applications of this technology, include:
- Ability to make a safe fuel.
- Simple electrochemical fuel metering.
Inventor Information
- Please review all inventions by Prof. Zachariah and his team, at UCR.
- Please visit Prof. Zachariah's research group website to learn more about their research.
- Please read recent press coverage of Prof. Zachariah, at UCR.
Patent Status
Patent Pending
Contact
- Venkata S. Krishnamurty
- venkata.krishnamurty@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
liquid fuels, volatility, flammability, electrochemical, fuel storage, fuel metering
