Unzipping Polymers For Enhanced Energy Release
Full Description
Background
Metal fuels such as aluminum (Al) have 3 times higher energy densities than the most powerful explosives - making Al particles the most commonly employed additive in energetic materials. Nanoscale metallic fuels have shown to be as much as 1000 times more reactive due to their high surface area to volume ratio. Unfortunately, due to significant sintering that occurs on a time scale often shorter than the combustion time, the initial nanoscale fuel is transformed into microscale particles. This increase in particle size slows down energy release rate and minimizes the advantages of employing nanoscale mtals. Strategies to mitigate this problem while showing considerable requires subsequent processing.
Technology
Prof. Zachariah and his team have developed a novel approach that utilizes a chain-unzipping polymer as a binder for energetic composites. The polymer (polypropylene carbonate - PPC) decomposes primarily through sequential monomer depolymerization. The strategy localizes the heat feedback to near the reaction front by driving the endothermic chemistry of unzipping. This then liberates the gas near the flame front and propels particles away from the burning surface to minimize agglomeration and sintering. The unzipping polymer decomposes into volatiles at a relatively low temperature which significantly reduces the sintering.
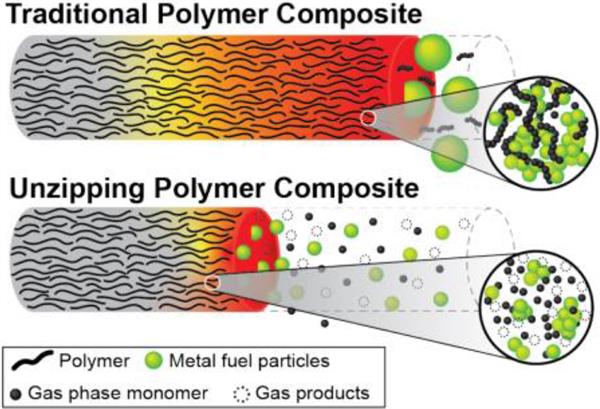
Conceptualization of unzipping versus traditional polymer binder.
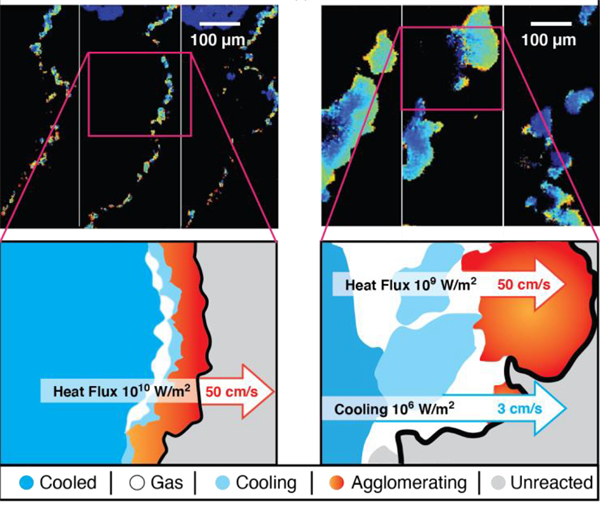
Flame front temperature map and corresponding schematic of composites with PPC (on the left) and HPMC/PVDF (on the right).
Advantages
The developed composite samples demonstrate:
- Significantly higher energy release rate - 15 times higher than conventional polymer binder mixture.
- Fast flame propagation speeds - 40 cms/seec compared to 3 cms/sec for the conventional mixture.
- A much thinner and continuous flame front with smaller particles with 10 times higher heat flux which significantly enhances the macroscopic flame propagating velocity to 50 cms/sec.
- A 14 times increase in gas production which significantly reduces agglomeration/sintering which dramatically increases flame propagation and energy release rate.
Suggested uses
Applications that use energetic materials such as propellants, solid fuels, thermites, etc.
Related Materials
Inventor Information
- Please see recent press coverage of Prof. Zachariah and his research at UCR.
- Please visit Prof. Zachariah's research group website to learn more about their research.
- Please review all inventions by Prof. Zachariah and his team at UCR.
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Published Application | 20240002310 | 01/04/2024 | 2022-863 |
Contact
- Venkata S. Krishnamurty
- venkata.krishnamurty@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
energetic materials, explosives, propellants, nanothermites, thermites, pyrotechnics, solid fuel, polymers
