Monoclonal Antibody Protease Inhibitors
Background
Critical for diverse biological processes, proteases represent one of the largest families of pharmaceutical targets. To inhibit pathogenic proteases with desired selectivity, monoclonal antibodies (mAbs) hold great promise as research tools and therapeutic agents. However, identification of mAbs with inhibitory functions is challenging because current antibody discovery methods rely on binding rather than inhibition.
Brief Description
Researchers from the University of California, Riverside have identified novel protease inhibitory mAbs including MMP-12, MMP-14, BACE-1, Alp2, and cathepsin mAbs. Matrix metalloproteinases MMP-12 and MMP-14 are involved in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. This technology is significant because these proteases precisely control a wide variety of physiological processes and thus are important drug targets for use in therapy for a wide range of diseases.
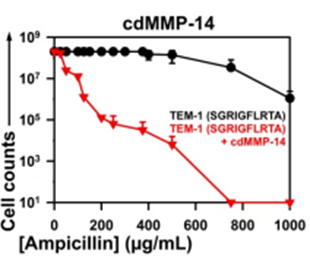
Fig 1: Selection windows for the UCR cdMMP-14 inhibitors. β-lactamase TEM-1 was modified by insertion of the protease specific cleavage peptide sequences (shown in parentheses) between Gly196 and Glu197 of TEM-1.
Suggested uses
- For use as potential therapies for a wide range of diseases including arthritis and metastasis.
- For use as a research tool
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Issued Patent | 11,332,546 | 05/17/2022 | 2019-764 |
Related Materials
Contact
- Grace Yee
- grace.yee@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
MMP-12, MMP-14, monoclonal antibodies, neuropathic pain, inhibitors
