Potent and Effective Anti-Metastatic EphA2 Agonists
Background
Ephrin receptor A2 (EphA2) is a member of a receptor tyrosine kinases and its overexpression is invariably associated with poor prognosis and the development of a variety of aggressive metastatic cancers. Although EphA2 is a compelling target, the antibody-drug conjugate (ADC), MEDI-547, failed clinical trials due to adverse side effects. The side effects included bleeding and liver dysfunction and this may be due to the long half-life of the antibody which drives the ADC metabolism and excretion through the liver, gall bladder and gastrointestinal tract.
However given the pivotal role of EphA2 in tumor growth, angiogenesis, cancer drug resistance, and metastasis, EphA2-targeting agents that are not ADCs or peptide-drug conjugates may be developed as cancer therapeutics or diagnostics with less side effects. In addition, agonistic EphA2 agents can be used as effective anti-metastatic therapeutics, inhibit cancer cell migration and invasion.
Brief Description
Prof. Maurizio Pellecchia and his colleagues at the University of California, Riverside have developed peptide-based EphA2 agonistic agents that have nanomolar activities. These agents, having the same mechanism of action as the natural (ephrinA1-Fc) ligands, effectively degrade EphA2 receptors and delay cell migration in key cancer cell lines. These agonistic agents may be effective therapeutics that may result in less unwanted side effects that have been observed in the clinic with ADCs targeting EphA2.
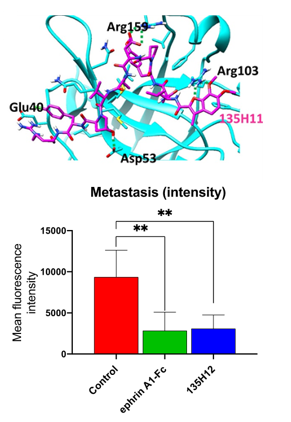
Fig. 1 Top, X-ray structure of EphA2 in complex with UCR agent.. Bottom, Treatment with ephrinA1-Fc or UCR agent 135H12 on an orthotopic mouse model of prostate cancer with PC-3-GFP cells (n = 5 mice per treatment group). The mean fluorescence intensity related to metastases detected at day 7 from mice in each group, control (the solvent formulation used for 135H12), ephrinA1-Fc treated, 135H12 treated. Error bars represent standard deviation. ** p < 0.01.
Application
Peptidomimetic agonists may be developed as cancer therapeutics, as targeted delivery agents for chemotherapy, or as diagnostics
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Issued Patent | 11,739,121 | 08/29/2023 | 2018-541 |
| Patent Cooperation Treaty | Published Application | 2019/237075 | 07/02/2020 | 2018-541 |
Related Materials
- Salem AF et al., Prostate Cancer Metastases Are Strongly Inhibited by Agonistic Epha2 Ligands in an Orthotopic Mouse Model. Cancers (Basel). 2020 Oct 2;12(10):E2854. doi: 10.3390/cancers12102854. PMID: 33023262. - 10/02/2020
- Salem AF et al., Therapeutic Targeting of Pancreatic Cancer via EphA2 Dimeric Agonistic Agents Pharmaceuticals 2020, 13(5), 90; https://doi.org/10.3390/ph13050090 - 05/10/2020
- Gambini L. et al., Structure-Based Design of Novel EphA2 Agonistic Agents with Nanomolar Affinity in Vitro and in Cell. ACS Chemical Biology 2018 13 (9), 2633-2644 DOI: 10.1021/acschembio.8b00556 - 08/15/2018
- Salem AF et al., Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide–Drug Conjugate. Journal of Medicinal Chemistry 2018 61 (5), 2052-2061 DOI: 10.1021/acs.jmedchem.7b01837 - 02/22/2018
Contact
- Grace Yee
- grace.yee@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
peptides, ephrin, EphA2, metastatic, pancreatic, prostate, ovarian, lung, breast, melanoma, agonist
