A Novel Catalyst for Aqueous Chlorate Reduction with High Activity, Salt Resistance, and Stability
Background
Both chlorate (ClO3-) and perchlorate (ClO4-) have negative effects on both human health and manufacturing process. In chlor-alkali plants (whose by-product is chlorate) the chlorate is currently remediated by either comproportionation reaction or catalytic reduction by H2. Catalytic reduction of ClO3− by platinum group metal catalysts and H2 gas allows clean conversion of ClO3− to innocuous Cl− (the only byproduct is H2O). However, practical applications of previously reported catalysts are challenged by:
- limited activity at ambient temperature and pressure;
- severe inhibition by concentrated salts in the brines; and,
- require high catalytic loadings to achieve a satisfactory reaction rate.
Brief Description
Inspired by biological systems, Prof. Jinyong Liu’s lab at UCR has developed a novel heterogeneous, bimetallic catalyst MoOx-Pd/C. The catalyst contains earth-abundant molybdenum (Mo) and the carbon support of Pd/C has a high capacity to accomodate MoOx species. The incorporation of a MoVI yields a highly active and robust catalyst. The porous carbon mimics the enzyme protein pocket (of microbes) to accommodate the oxygen atom transfer metal site. The representative figures shown below demonstrate the high activity and robustness of the catalyst for both chlorate and perchlorate reduction.
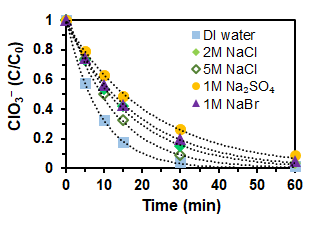
The effect of concentrated salts on the reduction of 1 mM ClO3− by the MoOx-Pd/C catalyst at a loading of 0.2 g/L. The reactions were conducted at 25 oC and under 1 atm H2.
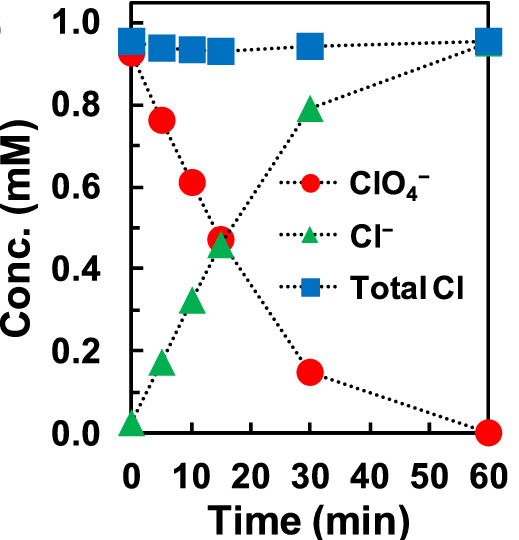
Chlorine balance for ClO4- reduction
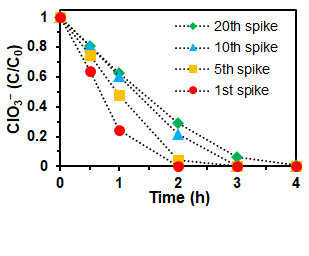
Fig. 3 shows the profiles of the reduction of 0.18M ClO3− spikes in a multiple-spike reaction series. The decrease of activity was only caused by the gradual build-up of concentrated Cl− (see details in the publication).
Advantages
- 55-fold more active than palladium on carbon (Pd/C). Under 1 atm H2 and room temperature, the (MoOx−Pd/C).
- Enables rapid and complete reduction of ClO3− in a wide concentration range (e.g., 1 μM to 1 M) and ClO4- concentration ranges from 10μM to 0.1 M.
- Exhibits strong resistance to concentrate salts such as chloride, sulfate, and bromide at 1 to 5 M.
- In a batch reactor setup, the catalyst was reused for twenty cycles of 0.18 M ClO3− reduction and no activity loss was observed.
Application
The high activity, outstanding stability, and strong resistance to common salts make the MoOx−Pd/C suitable for removing ClO3− and ClO4- and other oxyanions in the brine and in the chlor-alkali process and other scenarios such as water purification, wastewater treatment, and waste brine valorization.
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Issued Patent | 11,819,835 | 11/21/2023 | 2020-216 |
Related Materials
- A Bioinspired Molybdenum Catalyst for Aqueous Perchlorate Reduction
- Catalytic Reduction of Aqueous Chlorate With MoOx Immobilized on Pd/C
Related Technologies
Please review all water treatment technologies at UCR.
Contact
- Venkata S. Krishnamurty
- venkata.krishnamurty@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
Chlorate reduction, wastewater treatment, water treatment, chlor-alkali process, molybdenum, palladium, hydrogenation catalysts
