SPHERMAR(TM): a 3D High Throughput Screening Platform for Anticancer Drug Discovery and Development
Background
Cancer relapse and subsequent metastatic disease pose the most critical challenges in anticancer drug discovery and development. The limited clinical translational value of analyses performed on 2D cell cultures has prompted a shift toward the generation of 3-dimensional (3D) multicellular systems. One such 3D model system is the spontaneously-forming in vitro cancer spheroid model, referred to as spheroidsMARY-X, which precisely reflects the pathophysiological features of both tumor tissue and the lymphovascular embolus. However, these spheroids were not previously compatible with options for commercial high throughput drug screening.
Technology Description
By combining distinct features of the spheroidsMARY-X with a simple method for reading perturbation of the spheroid cohesiveness (i.e. dissolution index), the spheroid model has been transformed into a rapid, quantitative means to evaluate a drug’s dose-response curve. The screening ability of this platform, referred to as SPHERMARTM (see figure, below), is enabled by the observation that a drug’s induction of apoptosis correlates with the loss of well-circumscribed edges of the usually tight, compact spheroidsMARY-X where dissolution into a single cell population is consistent with cell death of the spheroid. Dissolution indices are determined through simple, brightfield image analysis of circularity (intact spheroid) vs. dissolution (single cell populations). The output of the SPHERMARTM platform is plotted as dose response curves which give reproducible and predictive IC50 values for anticancer drug screening and development.
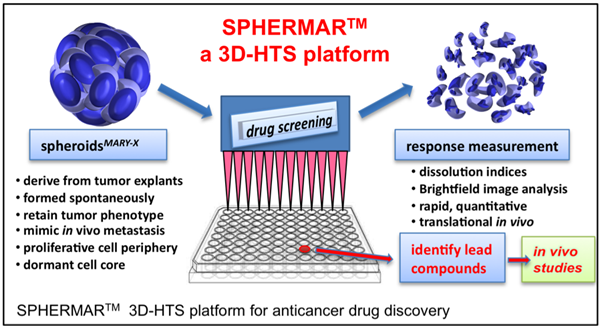
Applications
High throughput screening of drugs for relapsing and metastatic cancers.
Advantages
The SPHERMAR™ platform (Figure 1) has distinct advantages over other screening platforms since it creates high-throughput compatible 3D cultures that:
- are based on spontaneously-forming spheroids and thus do not require extraneous protocols to induce spheroidal morphology;
- retain the parent tumor phenotype and accurately mimic both in vivo metastasis (i.e. lymphovascular embolus) and the intratumoral biological complexities of the living tissue;
- are high yielding, simple (≤ 5 assay steps) and low cost;
- translate to a multiwall (>96) plate without the need for replacement or exchange of media or addition of molecular reagents to measure response; and
- produce reliable translational results in vivo
State Of Development
The SPHERMAR™ platform has been validated by in-house screening of two small-molecule libraries. Two promising anti-cancer compounds have been identified with IC50 values that correlate well to values derived from dual fluorescence assays.
Intellectual Property Info
Worldwide rights available; pending patents available under confidentiality. Issued US patent (6,998,513) is listed under “Patent Status”.
Related Materials
- Theodoraki, M.A., et al. Spontaneously-forming spheroids as an in vitro cancer cell model for anticancer drug screening, 2015, Oncotarget, 2015 Jun 18. [Epub ahead of print]PMID:26101913 - 06/18/2015
- http://theodorakisgroup.ucsd.edu/
Patent Status
Patent Pending
Contact
- University of California, San Diego Office of Innovation and Commercialization
- innovation@ucsd.edu
- tel: View Phone Number.
