Assay to Measure Huntington's Disease Progression and Response to Treatment
Background
Huntington's disease (HD) is a neurodegenerative genetic disorder caused by the aggregation of the mutant protein mHttn. HD progression occurs over many years and its symptoms progress at different rates for each patient. Researchers at UCI have developed a method for monitoring the severity of HD and its progression or remission in a subject. In addition, the invention provides further assays for the development of new therapeutic compounds for the treatment of HD. There are currently no accurate and inexpensive tests for monitoring the severity and progression of HD; the development of effective treatments will be greatly accelerated by such an assay.
Technology Description
Researchers at UCI have developed an assay to monitor the progression of Huntington's Disease utilizing a subjects' cerebrial spinal fluid (CSF). It was discovered that CSF from HD patients, when put into contact with cells expressing expanded polyglutamine Httn variants, has the ability to increase aggregation of the Httn variants within the cells. This activity, referred to as "seeding", is measured by the number of cells with aggregates and by the amount of aggregates within the cells. This enhanced aggregation is quantifiable in HD CSF and used as a measure of disease presence, HD progression or remission, including the effects of various HD therapeutic interventions. The below figure illustrates the assay.
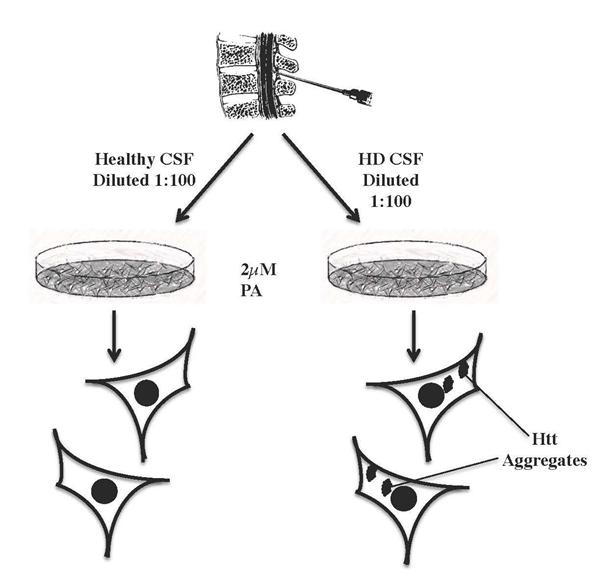
Schematic of assay. Cells in tissue culture plate express mHttn, after adding CSF, aggregation is measured after 16 and 24 hrs
Future Development Plans
Ongoing experiments will evaluate the correlation between enhanced aggregation in patients with pre-symptomatic and different stages of symptomatic HD to validate the assay. We are interested in partnering with companies that wish to utilize this assay in their HD drug development programs or to further develop the assay for clinical use.
Features/Benefits
- Monitors the severity and progression of Huntington’s disease.
- Allows improved determination of stage of disease or prediction of onset of HD symptoms.
- Provides assays for the development of new therapeutic compounds for the treatment of HD, including identification of patient populations that may be best suited for a particular drug or clinical trial.
- Assay is sensitive to progression in HD and more related to the etiopathology of HD than current imaging measures.
Publications
http://www.ncbi.nlm.nih.gov/pubmed/26100538 Huntington's disease cerebrospinal fluid seeds aggregation of mutant huntingtin. Mol Psychiatry (2015) 1-8.
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Issued Patent | 9,989,540 | 06/05/2018 | 2013-804 |
Contact
- Casie Kelly-Quintos
- casie.kelly@uci.edu
- tel: View Phone Number.
Inventors
- Glabe, Charles G.
- Potkin, Steven G.
- Tan, Zhiqun
- Thompson, Leslie M.
