Novel AMPK Inhibitors and Activators
Tech ID: 34072 / UC Case 2024-766-0
Background
The compound class of protoberberine alkaloids has been recognized for its potential as AMPK activators, thus positioning them as promising therapeutic agents for chronic metabolic diseases such as type-2 diabetes and obesity. However, emerging research has demonstrated a new aspect of these natural product protoberberine alkaloid derivatives: their capacity for AMPK inhibition. This suggests that protoberberine derivatives could also play a crucial role in treating conditions where AMPK inhibition is beneficial, including certain cancers, the regulation of autophagy, and elderly wasting.
Brief Description
Professor Kevin Kou and colleagues from the University of California, Riverside and the City of Hope National Medical Center have developed a chemical synthetic strategy that allows for the efficient generation of a diverse library of oxyberberine derivatives. This technology is advantageous because the family of protoberberine molecules, the best known being berberine, is generally considered non-toxic. As such, protoberberine derivatives are likely to elicit a better safety profile compared to existing AMPK inhibitors that are highly toxic and be developed to treat a range of diseases.
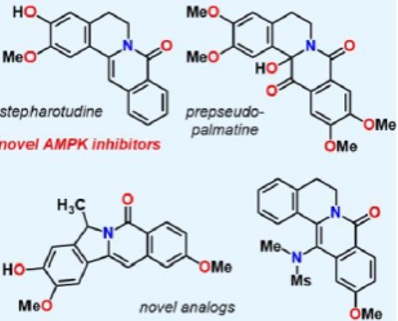
Fig 1: Four of the UCR novel AMPK inhibitors resulting from the UCR synthesis strategy.
Applications
- For the development of novel therapeutics ranging from cancer therapy to addressing metabolic imbalances and elderly wasting.
Patent Status
Patent Pending
Related Materials
Contact
- Grace Yee
- grace.yee@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
AMPK inhibitors, AMPK inhibition, AMP-activated protein kinase, protoberberines, protoberberine alkaloids
