Substantial defluorination of chlorinated PFCAs
Full Description
Background
Polychlorofluoroalkyl compounds represent a large group of per- and polyfluoroalkyl substances (PFAS). Well known examples include polychlorotrifluoroethylene (PCTFE) and chlorotrifluoroethylene (CTFE). The thermolysis of PCTFE and the metabolism of CTFE results in the formation of CTFE oligomer carboxylic acids which pose a risk to the hydrosphere. Prior research examining the biodegradation of chlorinated polychlorofluoroalkyl substances (Cl-PFAS) focused on a narrow range of compounds and identified reductive chlorination as the primary mechanism. There remains a significant gap regarding the biodegradability and ultimate fate of Cl-PFAS in the environment.
Technology
Prof. Yujie Men and her team investigated the biotransformation of Cl-PFAS by anaerobic microbes and have recently demonstrated that microbial dechlorination specifically hydrolytic dechlorination is a crucial step in the defluorination of CTFE oligomers. They also demonstrate that increasing the number of chlorine substitutions enhances their biodegradability leading to more extensive defluorination. The team shows that an anaerobic-aerobic treatment process could enhance the complete destruction of Cl-PFAS.
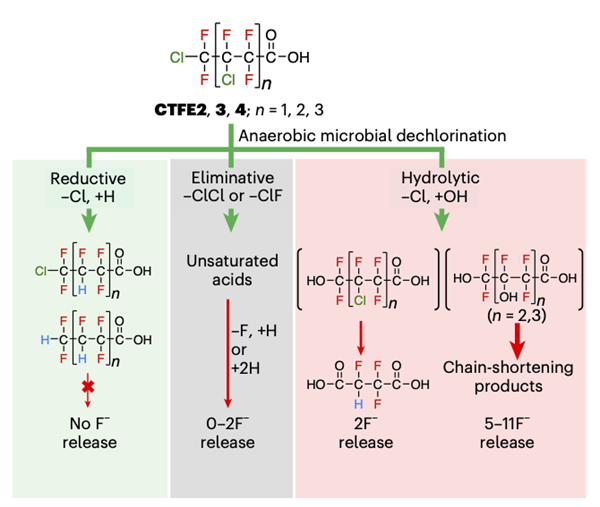
Image showing the biotransformation pathways. Green arrows represent dechlorinating reactions. Red arrows represent defluorinating reactions. The thickness of the red arrows represents the number of fluoride ions (F-) released - a metric for the extent of breakdown of carbon-fluorine (C-F) bonds.
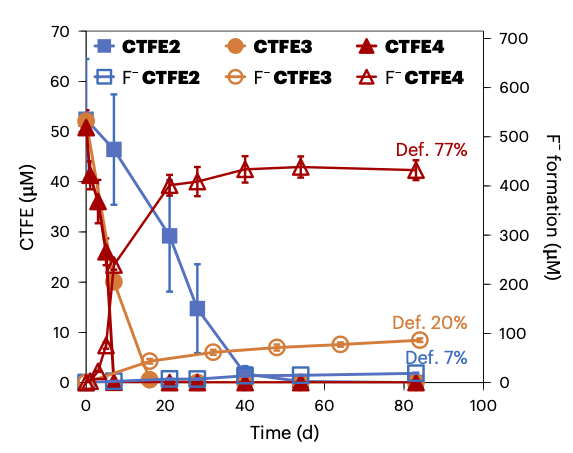
Graph showing the parent compound decay and F- formation. The data are represented as mean values. The error bars represent the standard deviation from n=3 replicate experiments.
Advantages
Cost-effective bioremediation - Sequential anaerobic-aerobic bioreactors, commonly used in wastewater treatment, could be adapted for a cost-effective cleanup of impacted environments.
Designing safer alternatives - Incorporating chlorine substitutions in PFAS molecules could offer a viable strategy for designing new PFAS substances with similar functions but a significantly reduced environmental footprint.
Understanding environmental fate - the technology can be used to predict the behavior and persistence of Cl-PFAS in anaerobic environments.
Enhanced PFAS source tracking - the technology developed can aid in tracking the origin and fate of PFAS in the environment.
Applications
- Cost-effective remediation of PFAS contamination.
- Design of safer PFAS alternatives.
Inventor Information
Please review all inventions by Prof. Men and her team at UCR.
Please visit Prof. Men's group website to learn more about their research.
Related Materials
Patent Status
Patent Pending
Contact
- Venkata S. Krishnamurty
- venkata.krishnamurty@ucr.edu
- tel: View Phone Number.
Other Information
Keywords
anaerobic, biodegradation, CTFE, Cl-PFCA, hydrolytic dechlorination, microbial dechlorination, PFAS, wastewater treatment
